MIT-designed nanoparticles communicate with each other inside the body to target tumors more efficiently.
For decades, researchers have been working to develop nanoparticles that deliver cancer drugs directly to tumors, minimizing the toxic side effects of chemotherapy. However, even with the best of these nanoparticles, only about 1 percent of the drug typically reaches its intended target.Now, a team of researchers from MIT, the Sanford-Burnham Medical Research Institute and the University of California at San Diego have designed a new type of delivery system in which a first wave of nanoparticles homes in on the tumor, then calls in a much larger second wave that dispenses the cancer drug. This communication between nanoparticles, enabled by the body’s own biochemistry, boosted drug delivery to tumors by more than 40-fold in a mouse study.
This new strategy could enhance the effectiveness of many drugs for cancer and other diseases, says Geoffrey von Maltzahn, a former MIT doctoral student now at Cambridge-based Flagship VentureLabs, and lead author of a paper describing the system in the June 19 online edition of Nature Materials.
“What we’ve demonstrated is that nanoparticles can be engineered to do things like communicate with each other in the body, and that these capabilities can improve the efficiency with which they find and treat diseases like cancer,” von Maltzahn says.
Senior author of the paper is Sangeeta Bhatia, the John and Dorothy Wilson Professor of Health Sciences and Technology and Electrical Engineering and Computer Science and a member of MIT’s David H. Koch Institute for Integrative Cancer Research.
Harnessing biology
Von Maltzahn and Bhatia drew their inspiration from complex biological systems in which many components work together to achieve a common goal. For example, the immune system works through highly orchestrated cooperation between many different types of cells.
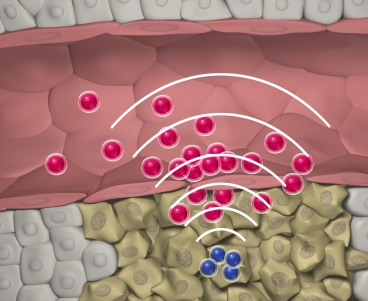 MIT researchers designed nanoparticles that can quickly locate a tumor, then set off a chemical reaction that attracts larger swarms of drug-delivering nanoparticles to the site.
Image: Gary Carlson
MIT researchers designed nanoparticles that can quickly locate a tumor, then set off a chemical reaction that attracts larger swarms of drug-delivering nanoparticles to the site.
Image: Gary Carlson
The MIT team’s approach is based on the blood coagulation cascade — a series of reactions that starts when the body detects injury to a blood vessel. Proteins in the blood known as clotting factors interact in a complex chain of steps to form strands of fibrin, which help seal the injury site and prevent blood loss.
To harness the communication power of that cascade, the researchers needed two types of nanoparticles: signaling and receiving.
Signaling particles, which make up the first wave, exit the bloodstream and arrive at the tumor site via tiny holes in the leaky blood vessels that typically surround tumors (this is the same way that most targeted nanoparticles reach their destination). Once at the tumor, this first wave of particles provokes the body into believing that an injury has occurred at a tumor site, either by emitting heat or by binding to a protein that sets off the coagulation cascade.
Receiving particles are coated with proteins that bind to fibrin, which attracts them to the site of blood clotting. Those second-wave particles also carry a drug payload, which they release once they reach the tumor.
In a study of mice, one system of communicating nanoparticles delivered 40 times more doxorubicin (a drug used to treat many types of cancer) than non-communicating nanoparticles. The researchers also saw a correspondingly amplified therapeutic effect on the tumors of mice treated with communicating nanoparticles.
To pave the path for potential clinical trials and regulatory approval, the MIT researchers are now exploring ways to replace components of these cooperative nanosystems with drugs already being tested in patients. For example, drugs that induce coagulation at tumor sites could replace the signaling particles tested in this study.
Jeffrey Brinker, professor of chemical engineering at the University of New Mexico, says the new strategy is a clever way to improve drug delivery to tumor sites. “Instead of targeting the tumor itself, it’s targeting a microenvironment that they’ve created,” he says. “By developing these nanosystems in a two-step approach, that could be used in combination with a lot of other strategies.”
Source: MIT News Release June 20, 2011
The originator of a press release or news release is responsible for it's content, not the UnderstandingNano Web site or Hawk's Perch Technical Writing, LLC
_______________________
Advertisments
_______________________
--------------------------
---------------------------

