Carbon Nanotubes
The discovery of carbon nanotubes happened in 1991. Carbon nanotubes had been known about since the 1950s, but were only understood sufficiently to make use of them in the 1990s. Today there are many nanotechnology companies pursuing nanotechnology research and producing nanomaterials.
Carbon nanotubes are composed of carbon atoms linked in hexagonal shapes, with each carbon atom covalently bonded to three other carbon atoms. A covalent bond is a chemical bond where electrons are shared. This bond forms electron pairs between the atoms called shared pairs or bonding pairs. These pairs maintain a balance of attractive and repulsive forces between the atoms that share the electrons. This balance is referred to as covalent bonding.
Carbon nanotubes are superstars when it comes to forming covalent bonds with other atoms because of something called electronegativity. This refers to the fact that carbon nanotubes can be used in a variety of settings because it’s relatively easy to covalently bond various atoms or molecules to nanotubes. This changes the chemical properties of the nanotube in a method called functionalization.
Where buckyballs are round, nanotubes are cylinders. That shape makes them more useful in applications which involve combining with other materials to improve strength or electrical properties. Electrical properties of carbon nanotubes are a result of how hexagons are arranged around a nanotube structure. This arrangement can affect whether the nanotube functions as a conductor or a semiconductor. The electrical resistance of carbon nanotubes can change when other molecules attach to them.
Carbon nanotubes have diameters as small as 1 nm and lengths up to several centimeters. Although, like buckyballs, carbon nanotubes are strong, they are not brittle. They can be bent, and when released, they will spring back to their original shape. Carbon nanotubes have several properties that make them useful: They are strong, elastic, lightweight, and conduct heat and cold well.
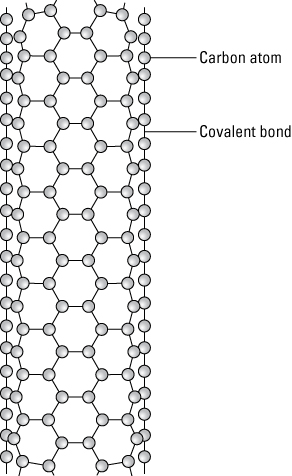
A carbon nanotube.
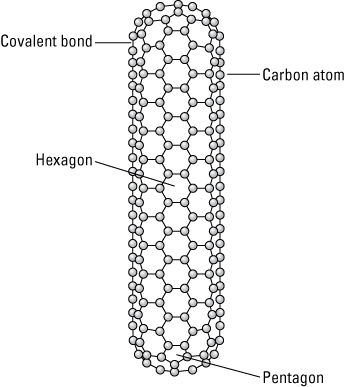
A carbon nanotube with closed ends.
Single-walled and Multi-walled Carbon Nanotubes
Carbon nanotubes can occur as multiple concentric cylinders of carbon atoms, called multi-walled carbon nanotubes (MWCNTs) as shown in the following figure. Logically enough, carbon nanotubes that have only one cylinder are called single-walled carbon nanotubes (SWCNTs). Both MWCT and SWCT are used in composite materials such as those used in nanotube-polymer composite blades used in windmills, or polymer-nanotube sensors activated when electrical resistance changes to indicate stress.
When MWCNTs are added to plastic they can increase electrical conductivity. SWCNTs do an even better job in this application because they can be applied to other materials than plastics. MWCNTs are longer than SWCNTs, and have a higher ratio of width to height (called an aspect ratio). They are much stronger than single walled carbon nanotubes (which are already strong in their own right). Multi walled carbon nanotubes are therefore used in applications where high mechanical strength is desirable. It’s also much easier to produce large quantities of MWCNTs. The problems encountered in producing SWCNTs on a large scale is reflected in their higher prices.
MWCNTs are longer than SWCNTs, and have a higher ratio of width to height (called an aspect ratio). They are much stronger than single walled carbon nanotubes (which are already strong in their own right). Multi walled carbon nanotubes are therefore used in applications where high mechanical strength is desirable. It’s also much easier to produce large quantities of MWCNTs. The problems encountered in producing SWCNTs on a large scale is reflected in their higher prices.But multi isn’t always better. When MWCNTs are added to plastic they can increase the material’s electrical conductivity, but SWCNTs increase the conductivity even more. This also works with materials other than plastic. SWCNTs don’t stop there: they have greater flexibility than MWCNTs. The multiple concentric layers in the walls of MWCNTs make them not only thick but they’re also quite stiff. An SWCNT is highly flexible, and even more flexible if found in smaller diameters. In fact, SWCNTs and are the best option for some applications.
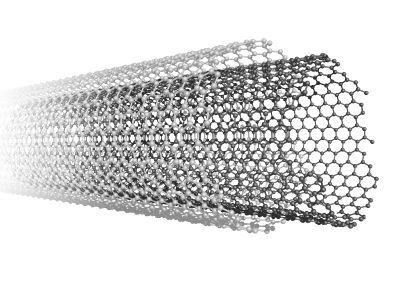
A multi-walled carbon nanotube.
Production of Carbon Nanotubes
There are different ways to produce MWCNTs. The first method that was discovered is referred to as “arc discharge”. A spark that arcs in the space between two graphite electrodes when experiencing high voltage, results in heat. This heat causes carbon in the positive electrode to be changed from a solid to a gas state. This then condenses on the negative electrode as CNTs.
Another method is called “laser ablation.” With this approach a pulsed laser vaporizes the carbon from a graphite target. A portion of the vaporized carbon forms into nanotubes as it condenses. At this point, the addition of metal catalyst particles makes the process more effective. The majority of the nanotubes produced by laser ablation are SWCNTs.
If you want to produce MWCNTs on a larger scale, the “chemical vapor deposition” (CVD) method is your best bet. This involves a hydrocarbon gas which is the carbon source for high-temperature decomposition producing CNTs. This method provides bigger yields and purer materials which saves on the cost of purification. Purification can’t be avoided for the arc discharge and laser ablation methods. You can also produce SWCNTs by CVD, but the you have to carefully control various conditions including temperature, the hydrocarbon source, the catalyst, and more.
Applications of Carbon Nanotubes
Because nanotubes have several valuable properties, researchers and companies to plan use them in several ways and settings making for a variety of nanotube applications. For example, researchers at NASA have used them in spacecraft because carbon nanotubes have the highest strength-to-weight ratio of any known material. By combining carbon nanotubes with other materials, they have produced composites that make more lightweight spacecraft. Nanotubes are also used in sensors that alert people to spoiling in food; in creating materials that absorb oil (for example from oil spills); in logic circuits or chemical sensors; and in anodes that can improve battery performance.
Other applications include:
- conductive latex gloves for touchscreen use
- antistatic conveyor rollers that dissipate the static charge
- in additives for conductive coatings
- to produce conductive ink for printing electronic components,
- in vehicle manufacturing to make components stronger and lighter.
- in carbon-fiber bicycle parts, where a resin with carbon nanotubes gain a greater strength-to-weight ratio.
- in the manufacture of sporting goods such as tennis racquets and golf clubs.
Sometimes combining properties offers new functionality. For example, you can reinforce silicon anodes for Li-ion batteries with CNTs which reduces their resistance and makes them more resilient to the cracking that occurs during thousands of charge/discharge cycles. SWCNTs do this even better than MWCNTs. That’s because the single-walled carbon nanotubes have high tensile strength and flexibility. This allows them to “bridge” particles, bringing them together and reducing cracking.
MWCNTs have high thermal conductivity which means that they can be used in materials to increase their ability to transmit heat. This characteristic can be in settings where heat needs to be dissipated, for example, in electronics. SWCNTs can provide a similar effect to conductive additives but they can do this in lower dosages, which reduces any negative effects on other properties of those materials.
Carbon nanotubes have some unique properties that make them useful in pharmaceutical research. For example, carbon offers strong adsorption capabilities. That ability enables a high loading capacity. Carbon nanotubes also have advantages as a result of their nanoscale channel structure, stability, adsorption property, and surface functionalization. These characteristics make them ideal for drug delivery systems where they’re used as drug carriers in loading drugs. Use of carbon nanotubes (CNTs) in drug delivery systems provide several benefits, including avoiding use of solvents and reducing side effects.
