Graphene; Discovery, Structure and Properties
Carbon can form three-dimensional lattices when it bonds with four other carbon atoms. This bonding forms diamond. Carbon can also form two-dimensional sheets by bonding to three other carbon atoms. These two-dimensional sheets are called graphene.
Discovery of Graphene
Graphene has had a surprisingly long history. The story of graphene goes way back to 1859 when a man named Benjamin Collins Brodie recognized a structure in thermally reduced graphite oxide. Jump forward to 1916 when the structure of graphene was determined by the use of powder diffraction. In 1924 the structure of graphene was further identified using single-crystal diffraction.
In 1947 P. R. Wallace proposed the theoretical existence of graphene in studies of the electronic properties of 3D graphite. Then, in 1986, three chemists by the name of Boehm, Setton, and Stumpp found that, within bulk materials, lay single layers of graphite seen inside soot obtained by chemical exfoliation. They coined the term graphene. (Graphene combines the word graphite with the suffix -ene, which refers to a bond in organic chemistry). Graphene is the term used for polycyclic aromatic hydrocarbons.
Researchers tried to make thin films of graphite using mechanical exfoliation beginning in 1990, but nobody could produce anything thinner than 50 to 100 layers before 2004. In 2004 single-layer graphene was produced by Andre Geim and Konstantin Novoseloy, crediting Peter Boehm who, in 1962, discovered graphene.
Production of Graphene
The National Graphene Institute, founded in 2014, supports applied research and development of graphene. They act as partners with various research groups and industry.
Once it was proved that production of graphene was possible at a commercial scale, commercialization of graphene developed quickly.Two manufacturers in northeast England, Applied Graphene Materials and Thomas Swan Limited, began manufacturing graphene. They worked in cooperation with Trinity College and various researchers. In 2017 following the creation of a laboratory graphene electronic device, a commercially viable integrated graphene electronics chip appeared. This chip was then marketed to pharmaceutical researchers.
Structure of Graphene
Though researchers only recently produced sheets of graphene for research purposes, they all probably had a handy form of graphene in their desk drawers. Common graphite is made from sheets of graphene in stacks, forming the material used in pencil lead. These sheets of graphene contained in graphite have a space between each sheet, as illustrated in the following figure. These sheets are held together by the electrostatic force known as van der Waals bonding.
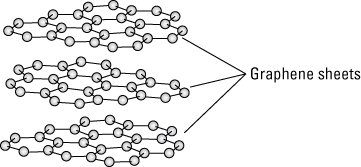
Sheets of graphene held together by van der Waals bonding make graphene.
Graphene sheets are composed of carbon atoms which are linked in hexagonal shapes, as shown in this figure. Each carbon atom is covalently bonded to three other carbon atoms. A sheet of graphene is one atom thick and each graphene sheet is therefore considered to be a single molecule. Graphene’s has the same carbon atom structure with links in hexagonal shapes as is used in forming carbon nanotubes, but graphene is flat rather than cylindrical.
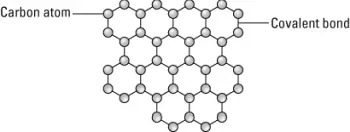
A graphene sheet
Properties of Graphene
The strength of covalent bonds between carbon atoms in graphene gives it a very high tensile strength. (tensile strength is essentially how much you can stretch something before it breaks.)
Graphene, unlike a buckyball or nanotube, has no inside. That’s because graphene is flat. Buckyballs and nanotubes, which have every atom on their surface, can only interact with molecules that surround them. With flat graphene, every atom is on the surface and so is accessible from both sides. That means that there is more interaction with surrounding molecules.
In graphene, carbon atoms are bonded to just three other atoms, but they do have the capability to bond to a fourth. This capability, along with graphene’s high tensile strength and its high surface area to volume ratio makes it useful in composite materials. It’s been reported that mixing graphene in an epoxy produced increased strength for the material equal to that found when using ten times the weight of carbon nanotubes.
An important electrical property of graphene is electron mobility (the speed at which electrons move within the material when you apply a voltage). N fact, graphene’s electron mobility is faster than that of any known material. Researchers are working on methods to build transistors on a graphene substrate that would be much faster than transistors currently built on a silicon substrates.
Because graphene takes advantage of the fact that the sheet is only as thick as a carbon atom, researchers have found that nanopores can be used to quickly analyze the structure of DNA. When a DNA molecule passes through a nanopore with a voltage applied across it, it’s possible to determine the structure of the DNA by changes in electrical current. Because graphene is extremely thin, the structure of a DNA molecule appears at a very high resolution when it passes through a nanopore that’s cut in a graphene sheet.
You can also read about graphene on the following webpages: