Nanotube 'sponge' has potential in oil spill cleanup
A carbon nanotube sponge that can soak up oil in water with unparalleled efficiency has been developed with help from computational simulations performed at the Department of Energy's (DOE's) Oak Ridge National Laboratory.
Carbon nanotubes, which consist of atom-thick sheets of carbon rolled into cylinders, have captured scientific attention in recent decades because of their high strength, potential high conductivity and light weight. But producing nanotubes in bulk for specialized applications was often limited by difficulties in controlling the growth process as well as dispersing and sorting the produced nanotubes.
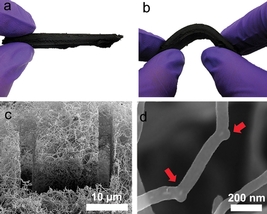 A carbon nanotube sponge developed with help from ORNL researchers holds potential as an aid for oil spill cleanup. Simulations at ORNL explained how the addition of boron atoms encouraged the formation of "elbow" junctions (seen in d) that help the nanotubes grow into large spongelike clumps (seen in a-c). The material is extremely efficient at absorbing oil in contaminated seawater because it attracts oil and repels water.
A carbon nanotube sponge developed with help from ORNL researchers holds potential as an aid for oil spill cleanup. Simulations at ORNL explained how the addition of boron atoms encouraged the formation of "elbow" junctions (seen in d) that help the nanotubes grow into large spongelike clumps (seen in a-c). The material is extremely efficient at absorbing oil in contaminated seawater because it attracts oil and repels water.
ORNL's Bobby Sumpter was part of a multi-institutional research team that set out to grow large clumps of nanotubes by selectively substituting boron atoms into the otherwise pure carbon lattice. Sumpter and Vincent Meunier, now of Rensselaer Polytechnic Institute, conducted simulations on supercomputers, including Jaguar at ORNL's Leadership Computing Facility, to understand how the addition of boron would affect the carbon nanotube structure.
"Any time you put a different atom inside the hexagonal carbon lattice, which is a chicken wire-like network, you disrupt that network because those atoms don't necessarily want to be part of the chicken wire structure," Sumpter said. "Boron has a different number of valence electrons, which results in curvature changes that trigger a different type of growth."
Simulations and lab experiments showed that the addition of boron atoms encouraged the formation of so-called "elbow" junctions that help the nanotubes grow into a 3-D network. The team's results are published in Nature Scientific Reports.
"Instead of a forest of straight tubes, you create an interconnected, woven sponge-like material," Sumpter said. "Because it is interconnected, it becomes three-dimensionally strong, instead of only one-dimensionally strong along the tube axis."
Further experiments showed the team's material, which is visible to the human eye, is extremely efficient at absorbing oil in contaminated seawater because it attracts oil and repels water.
"It loves carbon because it is primarily carbon," Sumpter said. "Depending on the density of oil to water content and the density of the sponge network, it will absorb up to 100 times its weight in oil."
The material's mechanical flexibility, magnetic properties, and strength lend it additional appeal as a potential technology to aid in oil spill cleanup, Sumpter says.
"You can reuse the material over and over again because it's so robust," he said. "Burning it does not substantially decrease its ability to absorb oil, and squeezing it like a sponge doesn't damage it either."
The material's magnetic properties, caused by the team's use of an iron catalyst during the nanotube growth process, means it can be easily controlled or removed with a magnet in an oil cleanup scenario. This ability is an improvement over existing substances used in oil removal, which are often left behind after cleanup and can degrade the environment.
The experimental team has submitted a patent application on the technology through Rice University. The research is published as "Covalently bonded three-dimensional carbon nanotube solids via boron induced nanojunctions," and is available online here: http://www.nature.com/srep/2012/120413/srep00363/full/srep00363.html.
The research team included researchers from ORNL, Rice University; Universidade de Vigo, Spain; Rensselaer Polytechnic Institute; University of Illinois at Urbana-Champaign; Instituto de Microelectronica de Madrid, Spain; Air Force Office of Scientific Research Laboratory; Arizona State University; Universite Catholique de Louvain, Belgium; The Pennsylvania State University; and Shinshu University, Japan.
The work was supported by the National Science Foundation, the U.S. Air Force Office of Scientific Research, the U.S. Army Research Laboratory, and by the DOE Office of Science through ORNL's Center for Nanophase Materials Sciences (CNMS) and the laboratory's Leadership Computing Facility.
CNMS is one of the five DOE Nanoscale Science Research Centers supported by the DOE Office of Science, premier national user facilities for interdisciplinary research at the nanoscale. Together the NSRCs comprise a suite of complementary facilities that provide researchers with state-of-the-art capabilities to fabricate, process, characterize and model nanoscale materials, and constitute the largest infrastructure investment of the National Nanotechnology Initiative. The NSRCs are located at DOE's Argonne, Brookhaven, Lawrence Berkeley, Oak Ridge and Sandia and Los Alamos national laboratories. For more information about the DOE NSRCs, please visit http://science.energy.gov/bes/suf/user-facilities/nanoscale-science-research-centers/.
Source: Oak Ridge National Laboratory News Release; May 10, 2012p>
The originator of a press release or news release is responsible for it's content, not the UnderstandingNano Web site or Hawk's Perch Technical Writing, LLC
--------------------------
---------------------------
